¹
²
International Journal of Epidemiology, Volume 33, Issue 3: Pages 542-548, June 2004.
¹ Department of Health Care and Epidemiology,
University of British Columbia, Vancouver, BC, Canada.
² BC Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada.
Accepted January 2004
Objective To observe recent trends in human immunodeficiency virus (HIV) prevalence in antenatal clinic attendees to determine if previously noted falls in HIV prevalence are occurring on both sides of the Kenyan-Ugandan border.
Design An ecologic study was conducted at the district level comparing HIV prevalence rates over time using data available through reports published by the Kenyan and Ugandan Ministries of Health and UNAIDS.
Methods Sentinel sites were compared with respect to population, ethnicity, language group, and the prevalence of circumcision practice. The prevalence of HIV found at each sentinel site was recorded for the years 1990–2000 and analysed visually and by conducting bivariate correlations.
Results Ethnographic analysis revealed a wide mix of ethnic and language groups and circumcision rates on both sides of the border. All sentinel surveillance sites in Uganda showed trends towards decreasing HIV prevalence, with three of five sites showing statistically significant declines (r = 0.87, 0.85, 0.86, P 0.05). In contrast, all of the surveillance sites in Kenya showed trends toward increasing HIV prevalence, with two of the five sites showing statistically significant increases (r = 0.62, 0.84, P 0.05).
Conclusions The declines in HIV prevalence occurring in Uganda are not being seen in geographically proximal districts of Kenya. No obvious differences in ethnic groupings or their associated prevalence of circumcision appeared to explain these differences. This suggests that decreasing HIV prevalence in Uganda is not due to the natural course of the epidemic but reflects real success in terms of HIV control policies.
Keywords Kenya, HIV, AIDS, prevalence, health policy, epidemiology
Despite the generally bleak picture presented by the devastating spread of human immunodeficiency virus (HIV) in Africa over the last decade, there is now evidence appearing that in some countries the size of the epidemic may be decreasing. Reports from Uganda and Zambia have shown declining prevalence rates in sentinel surveys of pregnant women since the mid-1990s, especially in younger age groups.1,2 In Uganda these decreases have been accompanied by concomitant delays in sexual initiation, decreases in casual sex among young people, and increased condom use.1 A recent study has now also described an observed decrease in HIV incidence in a cohort of nearly 7000 people in rural southwestern Uganda.3 Questions remain, however, as to what is driving these downward trends. It is possible that the decrease in Uganda simply shows that the peak (epidemic) prevalence of HIV is higher than the endemic prevalence of the disease and that this reflects only the maturing of epidemic. However, it may also be possible that these decreases reflect a real success of HIV control programmes in Uganda and that these programmes should be looked at more closely to provide lessons for other countries where HIV prevalence continues to increase.4
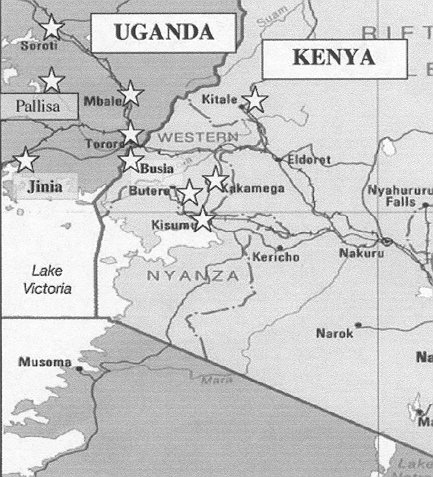
Kenya, with whom Uganda shares a border, has not seen these sorts of decreases in HIV prevalence.5,6 Estimates for the end of 2001 suggest that approximately 15% of the general population in Kenya is infected with HIV,5 versus only 5% in Uganda,7,8 which is much the reverse of the situation 10 years ago. Kenya has several sentinel surveillance sites which are situated in the Western highlands, close to Lake Victoria, which are in close proximity to the border with Uganda. Similarly Uganda has several sites in the eastern part of the country which are close to the Kenyan border, which share roughly the same geographical location. These sites represent a mix of ethnic and language groups on both sides of the border, and are located on the major transportation routes through the region. If the changes in HIV prevalence which are being seen for Uganda as a whole are due to natural changes in the course of the epidemic, one would expect to see them also occurring to some extent in neighbouring districts in Kenya. Therefore, we thought it useful to compare trends in HIV prevalence from sites on either side of this border, to get an indication if these changes are due to epidemiological or political (i.e. health policy) changes over the last decade.
Setting
Kenya and Uganda are countries in East Africa which have been known to be affected by generalized epidemics of HIV since the mid-1980s. Their common border begins on the shores of Lake Victoria and extends approximately 500 km to the border of Sudan. Kenya has an estimated population of 31 million people and Uganda has 24 million people.5,7 Both are categorized as low-income countries with GNP per capita figures of between 300 and 400. Both are ethnically diverse countries with more than 40 distinct ethno-cultural groups living within their borders.
Data sources
HIV surveillance data have been available for each country since the early 1990s. In the year 2000 Kenya had 2 urban sites and 14 rural sites reporting data and Uganda had 13 rural sites and 2 urban sites. Anonymous, unlinked prevalence surveys for HIV conducted once per year with antenatal clinic attendees has been recommended by UNAIDS as a relatively inexpensive way to acquire good epidemiological data on the course of the disease in low-income countries.9 The number of women tested in each site varies from year to year. In the year 2000, the Ugandan survey results were based on sample sizes of between 250 and 600 women per site.8 No sample size information was available from Kenya.
Sentinel surveillance sites in Kenya and Uganda from 1990 to 2000 were accessed through the UNAIDS web site.5,7 These data were compared with the most recently available national HIV/AIDS surveillance reports from each country to ensure accuracy and completeness.6,8 Five sites in eastern Uganda were chosen (Tororro, Mbale, Pallisa, Jinja, and Soroti) because of their proximity (<150 km) to the border with Kenya (Figure 1). All sites are located in the Eastern province of Uganda in the highland area, except for Jinja, which is located in Busoga province and is a port on Lake Victoria. Similarly, five sites within 150 km of the border were chosen from Kenya. Busia, Kakamega, and Mbale (Vihiga) are located in Western province. Kitale is in Rift Valley province and Kisumu is a port on Lake Victoria in Nyanza province. For comparative purposes, the median values for all rural sites in Kenya and Uganda were also analysed.
Simple population and ethnographic information was compiled for each site to ensure that there were no obvious confounding patterns between the Uganda and the Kenya group of sites which would lead to differences in HIV rates over time. Specifically, data on the district population, population of the principal town, major ethnic groups or groups and the language family of these groups were compiled.10-12 As well, information from published sources was compiled regarding the practice of circumcision in each ethnic group,13-17 as this has been shown to be associated with HIV prevalence in ecological studies.18
Data analysis
The percentages of all pregnant women who tested positive for HIV for each year were entered in Excel 98 (Microsoft Corp. Seattle, Washington) and graphed to show trends over time. The significance of these observed trend were then tested by conducting bivariate correlations to look for significant associations between increasing year and HIV prevalence for each site using SPSS software (SPSS Base 10.0, Chicago, IL) This method was chosen as we did not have sample size information which would have allowed us to perform a χ² test of trend.
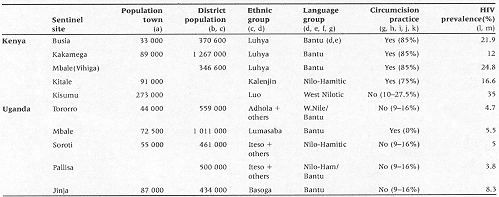
A summary of the population and ethnographic characteristics of the 10 surveillance sites is shown in Table 1. The Ugandan sites represented districts which varied in size between 434 000 people to just over 1 million. One site, Pallisa, did not have a principal town with over 15 000 people. The largest principal town of the Uganda sites was Jinja, the second largest city in Uganda, with just over 87 000 people. The districts sampled by these sites represent 12.4% of the Ugandan population. Similarly, the Kenyan sentinel sites represented districts of between 346 000 and 1.2 million people in size. One of the sites, Mbale (Vihiga), did not have a principal town larger than 15 000 people and the largest municipal population was that of Kisumu, Kenya's third largest city with 273 000 people. The districts sampled by these sites represent approximately 7.6% of the Kenyan population although it is probably more, given that we were unable to find reliable figures for the district populations of Kisumuor Kitale.
Data sources for Table 1
The Ugandan sites were composed of Busoga, Iteso, Adhola, Lumasaba, and other ethnic groups which belong to the Bantu, Nilotic, and Nilo-Hamitic language groups. Uganda as a whole has generally low rates of circumcision; approximately 9% in Kampala14 and approximately 16% in a recent study in the southwest.15 Only one of the groups involved in this study, the Lumasaba was described as having practised traditional circumcision.13 The Kenyan sites also represented a mix of ethnic groups, but were more clearly separated by sentinel site. The Luhya, a Bantu group, are the predominant ethnic group in Busia, Kakamega, and Mbale (Vihiga). The Luo, an Eastern Nilotic language group are the predominant group in Kisumu and the Kalenjin, a Nilo-Hamitic group are found in Kitale. As a whole, Kenya has high rates of circumcision, approximately 75–85%, and both the Kalenjin and Luhya people are known to use this practice.13,14 Luo people, however, do not traditionally practice circumcision resulting in overall circumcision prevalence of between 10 and 27.5%.16,17
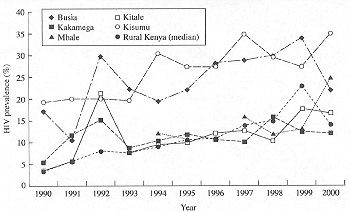
The trends in antenatal HIV prevalence for each of the five sites in Kenya and Uganda over time are shown in Figures 2 and 3. In the early 1990s there was no clear separation between the Kenyan and Ugandan sentinel sites. Both countries had sites with prevalence figures above and below 10%. However, by the year 2000, all of the Kenyan sites were reporting HIV prevalence figures above 10% and all of the Ugandan sites were now below 10%. Some of the data points vary greatly from year to year.
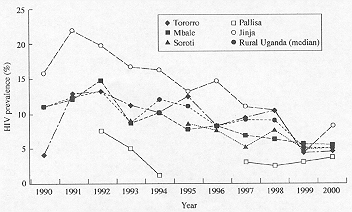
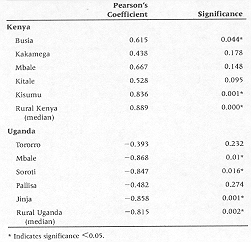
Bivariate correlations between year and HIV prevalence are shown in Table 2. All of the Kenya sites show trends towards positive correlation coefficients, suggesting increasing HIV prevalence figures over time. However, in only two of these sites, Kisumu and Busia are the correlations statistically significant (r = 0.84, P = 0.001; r = 0.62, P = 0.044). The trend for the median values from all rural Kenyan sites was also significantly positive (r = 0.889, P 0.001). By contrast, all of the Ugandan sites show trends towards decreasing HIV prevalence over time, with three of these (Mbale, Soroti, and Jinja) being statistically significant (r = 0.87, P = 0.01; r = 0.85, P = 0.016; 0.86, P = 0.001). The trend for the median values from all rural Ugandan sites was also significantly negative (r = –0.815, P = 0.002).
This study has shown that ethnically mixed populations of Kenya and Uganda who live in close geographical proximity to each other are experiencing significantly different recent trends in HIV prevalence in antenatal clinic attendees. These differences do not seem to result from clear differences in ethnic or language groupings and therefore can likely be assumed to be independent of any predominant risk behaviours which could predispose one or more groups to HIV infection. The trends also appear to be independent of the levels of circumcision in an ethnic group, a factor which previously had been used to explain the high prevalence of HIV found in East Africa.12 This therefore suggests that the decreasing prevalence of HIV seen in Uganda is not occurring as a natural course of the epidemic, but rather reflects real differences in HIV control policies between Kenya and Uganda.
Serial surveys of HIV prevalence in antenatal clinic attendees are a rather crude, but simple and inexpensive way to monitor the course of the epidemic. They inherently contain several biases which may change over time. Early in an epidemic, they tend to overestimate the prevalence of HIV in the population, as pregnant women are obviously more sexually active than the general population. As the epidemic matures and more women have become infected for longer periods of time, they will experience decreasing levels of fertility which in turn leads to HIV infected women becoming less likely to be selected for participation in serial surveys.4 Thus in a mature epidemic, antenatal surveys will tend to underestimate the extent of the epidemic. Given the close geographical proximity of these surveillance sites, however, it seems unlikely that these biases would be operating in opposite directions in Kenya and Uganda.
Another explanation for the decreasing prevalence seen in Uganda has been the maturing of the epidemic. That is, that the stable, or endemic prevalence is expected to be less than the epidemic prevalence of the disease. This could be because more people are dying from infections they acquired more than 10 years ago in Uganda and that people in Kenya have not lived with HIV for long enough for them to see this increase in mortality. Again, given the close geographical proximity and similarly ethnic heterogeneity of these communities, it seems unlikely that HIV arrived in these areas at a significantly different times in the past. This is especially true given that the prevalence of HIV in antenatal populations of these sites was essentially the same in Kenya and Uganda in the early 1990s.
These differences in rates could also be due to a decrease in the number of available susceptible people in the population over time as more of them have become infected in Uganda. However, HIV is different from many other epidemic diseases in that newly susceptible sub-populations emerge on a continual basis as young people become sexually active. If the pool of susceptible people is becoming smaller in Uganda, this can only be possible if the newly sexually active population has changed their behaviour to limit their risks for acquiring HIV. Published studies agree that this is probably happening in Uganda, and likely reflects some success of the Ugandan HIV prevention strategy, and not a natural course of the epidemic.1,19
The lack of an effect of circumcision prevalence on HIV prevalence is interesting, and runs counter to recent explanations of why HIV prevalence is so high in Kisumu.17 While Kisumu did have the highest prevalence of HIV found in any site among the lowest levels of circumcision, other high prevalence sites in Kenya, such as Busia have high levels of circumcision. As well, most of the Ugandan sites are home to ethnic groups where circumcision is not practised and their HIV prevalence rates have been decreasing. These findings do not suggest that circumcision does not offer some degree of protection from HIV infection, but rather demonstrates that reductions in the spread of HIV are possible even in populations with low circumcision rates.
So, if the size of the HIV epidemic is decreasing in Uganda, but not in Kenya, and it appears due to differences in policy, what types of policies may be responsible? Currently, both countries have national AIDS control commissions, which set policy for their respective HIV/AIDS programmes. However, the Ugandan AIDS Commission was established in 1986 within the office of the President, whereas it took until 1999 for a similar high-level body, the National AIDS Control Council to be established in Kenya.6,20 Thus, from the beginning of the epidemic there was a higher level of senior government commitment to combating HIV in Uganda. Through a combination of government and non-governmental organizations, Uganda has been more successful in developing preventive healthcare services for HIV, primarily voluntary counselling and testing (VCT), as well as services to provide care and support for those who test positive.21 The first AIDS Information Centre (AIC) opened in Kampala in 1990 in order to provide better access to VCT services. By 1993, AIC branches had been set up in three other centres, including two of the sites included in this study, Jinja and Mbale. As of 1999 AIC had provided counselling and testing to nearly 400 000 clients in Uganda through four independent sites and over 35 satellite sites in hospitals and health centres.21,22
In contrast, VCT for HIV in government health facilities in Kenya has only been widely promoted since 2001, beginning with the publication of national VCT guidelines. One of the largest VCT development programmes in Kenya, run by the US Centers for Disease Control, had provided VCT services to nearly 13 000 clients by the end of 2001.23 National figures for VCT services are not available, but access is generally considered tobe poor.24
VCT services in Uganda have also been supported by the efforts of The AIDS Support Organization (TASO), a non-governmental organization which has been providing social and psychological support and medical care to over 59 000 HIV positive clients since 1987 in Uganda. Jinja, Mbale, and Tororro all have TASO branches,21,25 whereas no such supportive organization exists in Kenya on such a scale.
As well, two large cohort studies have been operating in the south-west part of Uganda since the early 1990s, which may have raised the profile of HIV/AIDS in Uganda.3,15 In the study which showed declining incidence of HIV, the authors raised the possibility that the very existence of this project may have had an effect on reducing risk behaviour in their cohort.3 It is possible that this effect has been seen even in districts outside of the study area. Finally, the death of a prominent musician, Philly Lutaaya, who publicly announced he was suffering from HIV in 1988, probably has helped to raise awareness and decrease the stigma associated with HIV in Uganda.21,26 The Philly Lutaaya Initiative/People with HIV/AIDS Initiative was founded in his memory shortly after his death in order to help HIV positive people tell others about their condition.26 All of these initiatives likely have made the general population of Uganda more aware of the spread of HIV in their community and perhaps more responsive to risk reduction messages.
Both countries have developed public education programmes,6,19 but quiet
promotion and distribution of condoms, and school- and village-based health education programmes are thought to be widely available in Uganda,19 while their availability in Kenya is not known. Of note, in 1997, President Moi of Kenya stopped the planned implementation of a sex education programme in schools in response to pressure from religious leaders.27 No improvements in treatment services for sexually transmitted infections have been noted over the last 10 years in Uganda3 but have been reported for in two cities in Kenya, not included in this study.6
Whether a cause or an effect of these successes, Uganda has been more successful in securing funding for its efforts, as it had an estimated budget of $US 4.8 million for HIV/AIDS in 2001, versus $US 1.8 million in Kenya.28
This study has several limitations. Firstly, it assumes that serial surveys conducted in Kenya and Uganda followed the same methodologies and were free from systematic biases. However, we have no details about the data collection from each of these sites, except that these countries have agreed to participate in surveillance systems recommended by UNAIDS. The number of pregnant women surveyed for each year was not available, for either country. It is possible that smaller numbers being surveyed in Kenya could lead to less reliable results. It is likely that the number of women tested was set to provide reliable data for national or perhaps, regional surveillance, and may not be appropriate for a district-level analysis. However, it is unlikely that any differential biases would be observed so consistently over time.
Secondly, if there were reasons why HIV positive women would be deferred from attending antenatal clinics in Uganda or encouraged to attend antenatal clinics in Kenya, a systematic biasing of the data could result. For example, if prevention of mother-to-child transmission (PMTC) programmes were more widely available in Kenya than in Uganda, more women would probably opt for antenatal care in Kenya. However, there is no evidence that either of these possibilities have arisen. PMTC programmes have only become feasible on a large scale since the publication of the HIV NET 012 trial in 199929 and remain to be widely implemented in both countries.
Thirdly, it is still possible that significant differences exist in risk behaviours or other factors that protect against HIV transmission in the districts sampled in Uganda versus those in Kenya. These factors would need to have been in place before any government-sponsored interventions could begin to operate, and could have imposed a limit to the potential extent of the epidemic in these areas. Lower levels of multiple sexual partners, or polygyny could be such a factor. A lower prevalence of herpes simplex virus, type 2 (HSV-2) in the Ugandan population compared with Kenya could be another. The only published prevalence studies for HSV-2 in Kenya and Uganda found similarly high rates of infection in both countries, although the studies were conducted 8 years apart and may not be directly comparable.30,31 Trend data for HSV-2 infections are not available for either country.
Finally, this study assumes that the sexual behaviours and cultural practices are more similar within individual ethnic groups than that between different groups living in the same country. Further it assumes that populations living in each surveillance site reflect the behaviour and practices of the local dominant ethnic group. These generalizations ignore the mix of ethnic groups that live in each of the districts sampled, but seem reasonable in the absence of district-level information about these factors.
In summary, the Ugandan approach to dealing with HIV has involved many different players: community groups, non-governmental organizations, research groups, as well as government. While Kenya has also tried to develop a similar broad-based approach to HIV prevention and care, it appears to have started much later and has been less successful in disseminating its programmes and messages widely. While further research will help to define the differences in HIV services, programming, and risk behaviours between Kenya and Uganda, it seems unlikely that any single policy decision or programme will be able to explain the differences in HIV prevalence trends which we have observed. However, further research may be able to determine which aspects of the Ugandan approach have been most closely linked with falling HIV prevalence and incidence, and which aspects could be used as models for other countries in sub-Saharan Africa.
The authors wish to thank Dr Mark Tyndall and Dr Patricia Spittal for their suggestions during the writing of the manuscript, and Dr Steve Marion for his comments on the statistical analysis. Dr David Moore is supported by the Community Medicine Residency Program of the
University of British Columbia. Dr Bob Hogg is supported by an Investigator award from the
Canadian Institutes of Health Research and a senior scholar award from Michael Smith Health Research Foundation.
Correspondence: Dr David M Moore, Department of Health Care and Epidemiology, University of British Columbia, 5804 Fairview Ave, Vancouver, BC, Canada V6T 1Z3. E-mail: dmooretango@netscape.net
The Circumcision Information and Resource Pages are a not-for-profit educational resource and library. IntactiWiki hosts this website but is not responsible for the content of this site. CIRP makes documents available without charge, for informational purposes only. The contents of this site are not intended to replace the professional medical or legal advice of a licensed practitioner.
© CIRP.org 1996-2024 | Filetree | Please visit our sponsor and host:
IntactiWiki.