International Journal of STD & AIDS, Volume 10: Pages 8-16, January 1999.
Circumcised men are at greater risk of HIV infection
Summary: Thirty-five articles and a number of abstracts have been published in the medical literature looking at the relationship between male circumcision and HIV infection. Study designs have included geographical analysis, studies of high risk patients, partner studies and random population surveys. Most of the studies have been conducted in Africa. A meta-analysis was performed on the 29 published articles where data were available. When the raw data are combined, a man with a circumcised penis is at greater risk of acquiring and transmitting HIV than a man with a non-circumcised penis (odds ratio (OR)=1.06, 95% confidence interval (CI)=1.01-1.12). Based on the studies published to date, recommending routine circumcision as a prophylactic measure to prevent HIV infection in Africa, or elsewhere, is scientifically unfounded.
Keywords: Circumcision, HIV, risk factors for HIV infection, meta-analysis
In the late 1980s, several small studies conducted in Africa suggested an association between having a foreskin and a greater risk of contracting human immunodeficiency virus (HIV). Some of these studies based their conclusion by looking at maps, some looked at high-risk populations, and others looked at patrons of sexually transmitted disease (STD) clinics. Recently, however, several large random population surveys performed in Africa have found that circumcised men are more likely to be HIV infected. Despite inconsistent findings in the medical literature, there is a misconception that the foreskin places a man at greater risk for acquiring an HIV infection1 Thirty-five studies have been published in peer-reviewed journals that have addressed what role, if any, the prepuce plays in the transmission of HIV. Available data from these studies have been combined in a meta-analysis. Looking at the corpus of scientific literature concerning the relation of the prepuce with HIV may be helpful in developing recommendations for HIV prevention.
GEOGRAPHIC ANALYSIS
Three studies have linked the foreskin to HIV infection by looking at maps, instead of men. These studies found an association between the practice of male circumcision at a societal level and regional HIV seroprevalence in Africa. In locations where male circumcision is practiced, HIV seroprevalence was found to be considerably lower than in areas where it is not practiced2-4. Other cultural factors, such as age at first coitus, chastity and monogamy, that could otherwise explain the differences were not considered by the authors. The authors employed data collected in the 1950s concerning circumcision practices. They assumed that circumcision practices had not changed since that time and that, within a given area, circumcision was universal or completely absent. Circumcision was not a panacea in preventing the spread of HIV as several areas where circumcision was practiced also demonstrated high levels of HIV seroprevalence.
In all of these studies, the presence of other STDs and other risk factors were not taken into account. In addition, the indices of HIV prevalence were very crude, as were the data on which the circumcision rates were based.
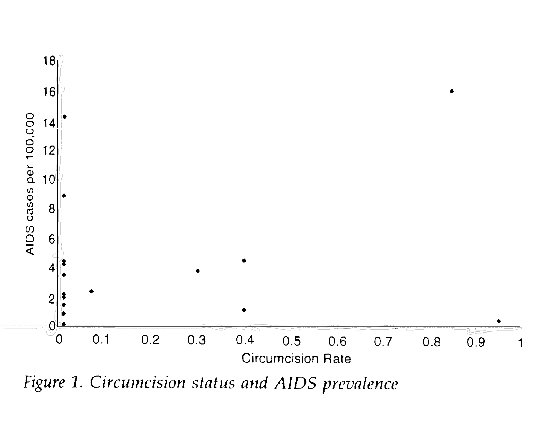
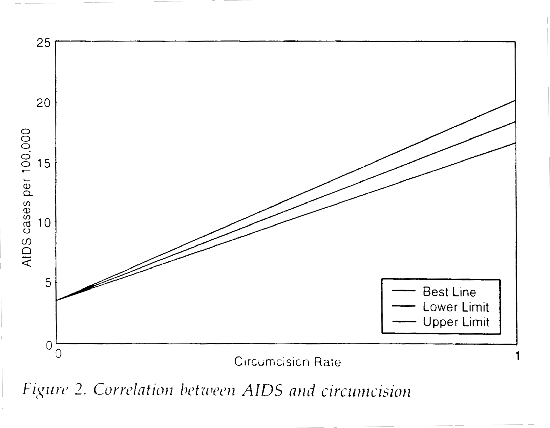
Applying the same methodology used in these studies to first world countries produces the opposite result. If the World Health Organization data from 1995 for AIDS prevalence5 are plotted against estimated circumcision rates (Table 1) and the data points are weighted for population, a positive correlation (Figures 1 and 2) is found between circumcision and AIDS prevalence (slope=14.95, 95% CI=13.19-16.71, R2=0.69) in first world countries6.
As with the previous map studies, this analysis ignores a number of important risk factors including cultural sexual practices. The results illustrate the inaccuracy and lack of power associated with this type of investigation.
Table 1. First world countries: circumcision rates, AIDS prevalence, and population
Population in
Circumcision AIDS prevalence thousands
Country rate(%) per 100,000 (1990)
Japan 1 0.2 123,638 Finland 1 0.9 4,984 Norway 1 1.5 4,247 Sweden 1 2.0 8,527 Germany 1 2.2 63,237 France 1 3.5 56,367 Mexico 1 4.2 88,598 Denmark 1.6 4.4 5,135 Italy 1 8.9 57,664 Spain 1 14.2 39,405 UK 7 2.4 57,410 Canada 30 3.8 26,560 New Zealand 40 1.2 3,296 Australia 40 4.5 17,083 USA 85 16.0 251,398 Israel 95 0.5 4,586
Most of the studies published have involved case-controlled studies or random population surveys, a summaryof which can be seen in Table 2.
Investigators have assessed high-risk patients by studying long-distance truck drivers, female sex workers, STD clinics, and tuberculosis patients.
Three studies looked at truck drivers who frequented the services of HIV-infected female sex workers7-9. All showed a significant association between the presence of a foreskin and a greater risk for HIV infection.
Most of the positive studies from Africa have been performed at STD clinics10-17, and have yielded mixed results. When comparing data collected by the same investigational group, findings to the degree described by Cameron et al.11, that men with foreskins were more likely to be HIV infected and more likely to acquire an HIV infection if not already infected could not be replicated in the study by Nassio et al.16, even though the Nassio study involved twice as many patients.

What consistently appears throughout all of these studies is the strong correlation between genital ulcer disease and HIV infection. There is also a correlation between genital ulcer disease and the foreskin in many of the studies. However, when multivariate analysis was performed on several of the studies, the foreskin as a factor was no longer significant12,13, suggesting that the genital ulcer disease rather than the foreskin facilitatedthe transmission of HIV.
Despite receiving a lot of attention following its publication in The Lancet, several methodological limitations are present in Cameron's analysis. For example, a selection bias may have resulted from a disproportionate number of circumcised men being lost to follow-up18. A substantial proportion of seroconversions may have been missed by including patients with only 2 weeks of follow-up19. If the proportion of missed seroconversions differed between circumcised and non-circumcised men, further bias may have been introduced.
In STD clinic studies conducted in the United States, there have been two abstracts and 2 studies published. The results have been mixed. While one abstract found an association between the foreskin and HIV infection20, the other did not.21 Similarly, the studies published in peer-reviewed journals included one study that showed no significance22, and one that did.23 In the one that did, the investigators relied on patient report to determine circumcision status. While the authors felt that it was unlikely that substantial misclassification occurred, reported positive predictive values of a male stating his penis is circumcised have been 35.5%24, 41.5%25, and 69.4%26. If the 41.5 positive predictive value is applied to this patient population, the correlation becomes smaller and non-significant (OR=1.15, 95% CI=0.79-1.68). Compared to the other risk factors studied, the foreskin was a minor player.
The two studies from STD clinics in India both found that the foreskin was associated with HIV infection27,28, but the differences were non-significant in one study28.
A study population and controls derived from the clientele of STD clinics do not reflect the population as a whole and introduce a population bias that may be in part responsible for the results seen. To generalize the results of studies conducted in STD clinics, one must assume that circumcised and non-circumcised men use these health facilities in the same manner. This, however, is not the case as socioeconomic and cultural factors often differ between the two groups. It is possible that circumcised men exhibit different health-seeking behaviour than non-circumcised men. If, for example, circumcised men are more likely to seek care for minor genital symptoms, they would be over-represented in the control population. This would cause the association between the foreskin and various STDs including HIV infection to be overestimated, if not spuriously generated29.
In a study in Abidjan, Côte d'Ivoire, of men diagnosed with tuberculosis, lack of circumcision was found to be a significant risk factor30.
Partner Studies were performed to see if any attributes of HIV-infected women were contributing factors31-36. Most failed to show the foreskin to be a significant factor (Table 2).
The most reliable study design is the random population survey. Several of these have been performed in Africa,26,37-46 with several of the studies demonstrating the circumcised male to be at greater risk for HIV infection (Table 2).
In an attempt to put these conflicting data into perspective, a meta-analysis was performed. Data was taken from all of the studies published in peer-reviewed medical journals. Two published studies provided their conclusions without providing data. One study found no significant association41, one did9. Two of the included studies equated being Muslim with being circumcised44,45, although religious preference in Africa does not always correlate with circumcision status.7,43 Another equated the presence of a long residual foreskin, following circumcision, with not being circumcised14. Although these may be erroneous assumptions, the inclusion or exclusion of these studies did not affect the overall outcome. The data are presented in Table 2. The combined data from the studies looking at high-risk populations found non-circumcised males to be at greater risk (OR=1.18, 95% CI=1.26-1.59), but only 11.7% of HIV infections could be attributed to the presence of the foreskin. Combining the partner study data yielded similar results (OR=1.42, 95% CI=1.26-1.59), while combining the random population surveys found non-circumcised men to be at significantly lower risk of acquisition or transmission of HIV (OR=0.94, 95% CI=0.77-0.97). When the raw numbers are accumlated from all of the published studies, having a foreskin significantly decreases the risk of acquisition or transmission of HIV (OR=0.94, 95% CI=0.89-0.99), although the impact is small. The weakness of applying the raw numbers in such a fashion are that the studies with large numbers dominate, and confounding factors cannot be accurately accounted for.
When studies are grouped based on the prevalence of circumcision in the community (excluding those studies using religion or degree of circumcision as a substitute for circumcision status), non-circumcised men were more likely to contract HIV if they were in the minority (OR=3.06, 95% CI=2.72-3.46). However, if non-circumcised men were in the majority, the presence of the foreskin was not a risk factor (OR=1.01, 95% CI=0.93-1.11). Minority groups are often at higher risk for disease because of economic disadvantages and increased exposure. this result reflects this well recognized trend.
Odds Relative Attributable
Author Intact+ Intact- Circ+ Circ- ratio 95% CI risk risk(%)
Truck drivers Bwayo7 92 86 160 612 4.09 2.91-5.76 2.49 59.9 Bwayo8 22 46 37 200 2.59 1.39-4.79 2.07 51.7 STD Clinics Kreiss23 59 18 254 168 2.17 1.27-3.81 1.27 21.4 Hira15 418 172 10 10 2.43 0.99-5.94 1.42 29.4 Cameron11 18 61 6 208 10.23 3.89-26.90 8.13 87.7 Pépin**14 5 42 13 243 2.22 0.75-6.57 2.09 52.3 Greenblatt12 11 28 8 68 3.34 1.21-9.18 2.68 62.7 Diallo10 38 46 212 873 3.40 2.16-5.36 2.32 56.8 Simonsen13 17 70 21 232 3.40 1.34-5.37 2.35 57.5 Tyndall17 85 93 105 527 4.59 3.20-6.58 2.87 65.2 Nasio16 86 78 137 580 4.67 3.26-6.68 2.74 63.6 Mehendal27 837 3411 38 253 1.63 1.15-2.32 1.51 33.7 Bollinger28 50 241 1 14 2.90 0.37-22.60 2.58 61.2 Chiasson22 36 797 14 542 1.75 0.93-3.27 1.72 41.7 TB patients Sassan30 75 18 415 221 2.22 1.29-3.81 1.24 19.1 High Risk total 1849 5207 1431 4751 1.18 1.09-1.28 1.13 11.7 Partner studies Hunter31 43 330 165 3765 2.97 2.09-4.24 2.75 63.6 Carael33 90 105 34 45 1.13 0.67-1.92 1.07 6.8 Chao34 442 4844 75 232 0.28 0.21-0.37 0.34 65.8+ Moss32 24 16 12 17 2.13 0.80-5.62 1.45 31.0 Allen35 275 612 132 324 1.10 0.86-1.41 1.07 6.6 Sedlin36 32 26 33 40 1.49 0.75-2.98 1.22 18.1 Konde-Luc*45 153 1516 6 127 2.13 0.93-3.27 1.72 41.7 Partner total 1059 7449 457 4550 1.42 1.26-1.29 1.36 26.7 Random population surveys Barongo37 55 1356 42 642 0.62 0.41-0.94 0.63 36.5+ Grosskurth38 158 4604 61 1026 0.58 0.43-0,78 0.59 40.9+ Van de Perre40 46 224 6 26 0.89 0.35-2.28 0.91 9.1+ Seed43 171 422 51 192 1.52 1.07-2.17 1.37 27.2 Malamba*4 111 114 21 47 2.17 1.22-3.88 1.60 56.8 Quigley46 101 272 48 121 0.94 0.62-1.40 0.95 4.7+ Urassa 126 56 1301 42 600 0.61 0.41-0.93 0.63 36.9+ Urassa 2 105 2040 32 426 0.69 0.46-1.03 0.70 29.9+ Urassa 3 38 309 19 158 1.02 0.57-1.83 1.02 2.0 Urassa 4 112 716 54 692 2.00 1.43-2.82 1.87 46.5 Urassa 5 101 365 48 136 0.78 0.53-1.16 0.83 16.9+ Random total 1054 11723 424 4066 0.86 0.77-0.97 0.87 12.6+ Total 3962 24379 2312 13367 0.94 0.89-0.99 0.95 5.2+
*Report Muslims versus non-Muslims
**Circumcised men with long residual foreskins were considered 'uncircumcised' by the authors
+Attributable risk of the circumcised penis
Intact+=non-circumcised and HIV+, Intact-=non-circumcised and HIV-, Circ+=circumcised and HIV+, Circ-=circumcised and HIV-, CI=confidence inteval, TB=tuberculosis
A casual relationship cannot be inferred from retrospective studies. To determine the likelihood of causality, Hill developed a number of criteria, which, if met, make a causal relationship more likely. Before intervention can be deemed warranted, it is important to determine whether the epidemiological data meet Hill'scriteria47 for causality.
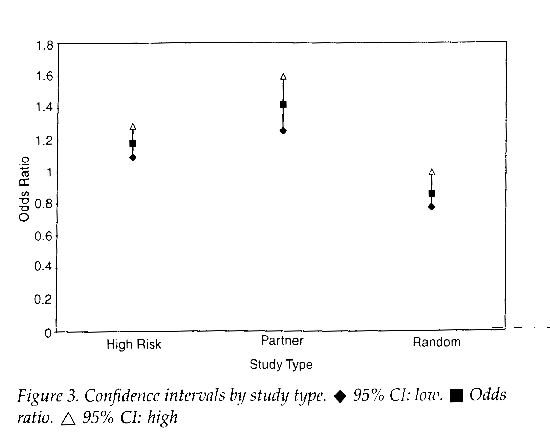
The relationship between the presence of a foreskin and HIV infection is strong is some studies, but the exact opposite in other studies. The design of the study is paramount, as non-causal associations can easily arise in observational studies due to confounding and misclassification48,49. The least reliable design study is the population-based random cluster sample survey. The difference in outcomes based on study design are shown in figure 3. Of the studies with most reliable study design, most found circumcised men to be at greater risk for HIV infection.
The results of the studies have varied markedly based on study design, time of study, and study location. Even utilizing similar designs has yielded discordant results. This strongly suggests that other factors associated with the foreskin, rather than the foreskin itself, are responsible for the acquisition and spead of HIV.
The multiple identified factors associated with the acquisition and transmission of HIV make it impossible to single out the foreskin as a significant factor. In numerous studies, the significance of circumcisionstatus is altered when other factors are controlled for.
Circumcision usually takes place before sexual activity is initiated. Only one study examined the timing of circumcision and its effect on HIV infection. When compared with men who were never circumcised, those circumcised after 15 years of age had a lower HIV prevalence (OR=0.48, 95% CI=0.25-0.90), while those circumcised before 15 years of age had a greater HIV prevalence (O=1.34, 95% CI=0.64-3.00)46. No explanation for this finding is given.
This involves demonstration of a dose-effect relationship. Only one study has looked at the degree of circumcision. In that single study, men with more residual skin were at greater risk of HIV-2 infection14. It is impossible to reach any conclusions based on that single study.
Moses et al. have suggested the following: (1) that minor inflammatory conditions occur underneath the foreskin resulting in mucosal discontinuity providing a portal of entry for HIV; (2) that the foreskin may be more susceptible to minor trauma during intercourse; (3) that the warm, moist environment under the foreskin provides an environment conducive to prolonged viral survival; and (4) that Langerhans cells, plentiful in the foreskin of male macaque monkeys, are highly susceptible to simian immunodeficiency virus (SIV)50.
While it has long been postulated that the warm, moist environment under the prepuce is a breeding ground for pathogens, the immunological protection provided by sub-preputial flora51, secretory immunoglobulins, and lytic secretions from the prostate, urethra, and seminal vesicles52 has not been adequately investigated, and may explain why American men with foreskins are less likely to have genital herpes53 and genital warts54.
The role of Langerhans cells is confusing. These cells initiate the immune response to infectious agents. While they are present in the prepuce of monkeys and adult males, Weiss et al. were not able to demonstrate their presence on the inner surface of the prepuce in tissue taken from the newborn penis55. Histological examination of the prepuce of older males has demonstrated Langerhans cells in the mucosa56. The physiological fusion of the preputial and glans mucosa and the sterile intrauterine environment may explain their absence in the newborn.
In the primate study referenced by Moses et al. it was Langerhans-like cells in the lamina propria, and not the epithelium that appeared to be infected with SIV57. It is unclear whether this observation can be extrapolated to the Langerhans cells in the epithelium of the prepuce in humans. More research is needed.
Beaugé suggests that the loss of the penile skin from circumcision can result in the skin being tight over the penis when erect. This tight skin increases the friction during intercourse and increases the likelihood of abrasions through which a virus can be introduced systemically. This may make the circumcised penis more likely to contract HIV58 The increased likelihood of circumcised men engaging in active anal sex53 may make the circumcised penis more likely to contract HIV infection.
Both theories evolve around skin and mucosal integrity. The presence of genital ulcers has been repeatedly demonstrated to be a more important factor than circumcision status.
The theory should fit in with other known characteristics of the illness. For example, in some cultures circumcision was once part of the coming of age ritual during which the boy is taught how to relate to women and how to maintain discipline in sexual matters. This teaching may have helped reduce high risk behaviours. Now the teaching has disappeared59. Instead, as a proof of manhood, sexual intercourse often follows soon after circumcision. This often takes place in the commercial sex market with a circumcision wound that has not completely healed60. Circumcision once fit a coherent social pattern of restraint and has been replaced with high risk behaviour. Currently in Africa, circumcised men may have more sexual partners17,26 and are less likely to be currently married (OR=0.7, 95% CI=0.5-0.9)17.
Tyndall et al. reported that the number of sexual partners and sexual exposure to female sex workers were not risk factors for HIV infection. The authors indict the foreskin as the culprit for HIV infection17. These findings are counterintuitive and contradict the corpus of the medical literature. An alternative explanation exists. The study only looked at men with genital ulcers. In men who present with an STD, circumcised men are more likely to present with a genital discharge while non-circumcised men are more likely present with genital ulcerations. The most common cause of ulcerations in Africa is chancroid. While chancroid has never been shown to be more common in non-circumcised men, it has a very high prevalence among certain ethnic groups that are usually economically depressed minorities61. The National Health and Social Life Survey published by investigators from the University of Chicago documented that sexual partners are not found randomly but from within one's social network, which is constrained by race, religion, ehnicity, and socioeconomic status. Additionally, they confirm that an STD with a high transmission rate will often spead very quickly and attain a higher prevalence rate within a smaller social network62 The decision by Tyndall et al. to implement their study design with a population where the minority of men were non-circumcised virtually guaranteed that non-circumcised men would be at higher risk for HIV infection. With a high infectivity rate, one could easily anticipate that chancroid, a highly contagious risk factor for HIV infection, could reach a higher prevalence in a minority non-circumcised population. Being a member of this portion of the population, not the foreskin, could be enough to negate known risk factors for HIV infection.
There is none.
Is there another illness to which the foreskin makes a man more susceptible? Can it be equated with the acquisition and transmission of HIV? The analogy of STDs has been used in the past, but recent studies have cast serious doubt on whether the foreskin is a factor in STDs53,54,63-65. Urinary tract infections in infancy may be associated with foreskin, but demonstrating how they would be analogous to HIV infection would be difficult.
Based on the above criteria, a causal relation between the foreskin and HIV infection cannot be demonstrated.
The quantification of a potential benefit, if any, that could be expected from male circumcision as protection from HIV transmission is highly problematic. Inconsistent study results coupled with the results of the meta-analysis emphasize this. While meta-analysis is an inexact tool66, it may help place the impact of small studies in perspective. Interestingly, the combined data from the high-risk populations yielded a lower odds ratio than any of the studies of that type. The lack of parity of circumcision status in the individual studies and the wide variation in HIV prevalence can help to explain this finding. By combining the data, the number of circumcised subjects is closer in number to those not circumcised than in any of the individual studies. Whether this makes the comparison more valid isdebatable.
Another weakness of the meta-analysis is the inability to correct for confounding factors, the most important of which is the presence of genital ulcers. It is also difficult to incorporate studies of varying design. Recent studies, in which the raw numbers suggest circumcised men to be at greater risk for HIV infection have found that circumcised men have more sexual partners17,26. When corrected for number of partners, the foreskin was found to increase the likelihood of HIV infection. Based on the recent findings that circumcision significantly alters sexual activity in adult males53, it may be inadvisable to look at these factors separately. It is likely that circumcision may be responsible for the increased numberof partners and therefore the increased risk.
The recommendation to routinely circumcise boys in Africa is unfounded and even dangerous. In some parts of Africa, circumcision is a leading cause of tetanus (59.4% of cases)67. The use of dirty instruments and mass ritual events, including group circumcision, may increase the number of young boys developing HIV infections68 The risk of spreading tuberculosis through circumcision in developing countries is also a valid concern69. Severe complications and death are not uncommon following ritual circumcision70.
If one assumes that circumcision does not prevent some cases of HIV infection, what impact would universal circumcision have? Using the data provided by Seed et al.43, the relative risk of developing HIV infection is 1.37 times greater in the male with a foreskin, and 27% of HIV cases might be attributed to this factor. With an AIDS prevalence in the United States of 16 per 100,0005 and an attributable risk of 27%, it would take 23,148 circumcisions to prevent one case of AIDS. In Australia and the UK, it would take 82,304 and 154,320 circumcisions respectively to prevent one case of AIDS. One could expect 46, 165, and 308 life-threatening complications in the US, Australia, and UK respectively, for each case of AIDS prevented72. In a developing country, the risks of tetanus, tuberculosis, infection, exsanguination, amputation, and death from circumcision would outweigh the benefit of preventing a small number of HIV infections.
The large discrepancies between the studies suggest that the foreskin is not a significant factor. Faced with a similar discrepancy between gonorrhoea as a risk factor for HIV infection in different geographical regions, Weir et al. concluded that gonorrhoeal infection did not facilitate HIV acquisition, but that having gonorrhoea acted as a marker for unprotected intercourse that facilitated HIV acquisition73. In similar fashion, the foreskin may be acting as a marker for othermore relevant factors.
For example, there may exist a causal relation between several STDs and HIV infection74,75, resulting from the mutual dependence of HIV and STDs on patterns of sexual activity or from the effect HIV may have on the clinical course of the STD. Likewise, the differing role of the prepuce as a risk factor, depending on the prevalence of circumcision in a region, suggests that the prepuce is a marker of the lack of socioeconomic and political privilege that factors heavily in the transmission and treatment of infectious diseases. The design of the study is paramount as non-causal associations can easily arise in observational studies due to confounding and misclassification48,49. Unfortunately with circumcision, interventional trials are not feasible to measure the effect of this variable. Until this interaction between STDs and HIV is understood and controlled for, the role of the prepuce cannot be adequately delineated.
Based on the studies published in the scientific literature, it is incorrect to assert that circumcision prevents HIV infection. Even if studies showing circumcision to be beneficial are accurate, the risk from circumcision outweighs any small benefit it may have. To depend on circumcision to protect against HIV infection in lieu of condoms, which have been shown to be efficacious76,77, is dangerous. Promoting circumcision as protection against HIV could also promote, intended or not, the inference, that a circumcised penis is adequate protection from contracting HIV, resulting in an increase in HIV infections. The circumcision experiment in the United States, which has failed to prevent the spread of this pandemic, should serve as a lesson to other countries.
American men are reluctant to use condoms. Studies indicate a considerably higher acceptance and usage rate for condoms in Europe and Japan, where circumcision is almost never practised. Some have suggested that American men are resisting a layer of latex that would further decrease sensation from a glans already desensitized from the keritinization following circumcision. Moreover, condoms are more likely to fall off the circumcised penis78. This low acceptance of condoms may be responsible for the high rate of STD and teenage pregnancy rates in the United States--the only industrialized country that has failed to control bacterial STDs during the AIDS era79.
Wise allocation of our resources demands that we divert our attention away from treating unproven risk factors and focus on proven prophylactic interventions.
Correspondence to Robert S. Van Howe, 9601 Townline Road, PO Box 1390, Minoqua, Wisconsin 54548-1390, USA. E-mail: mailto:vanhower@gabby.mfldclin.edu
(Accepted 3 June 1998)
The Circumcision Information and Resource Pages are a not-for-profit educational resource and library. IntactiWiki hosts this website but is not responsible for the content of this site. CIRP makes documents available without charge, for informational purposes only. The contents of this site are not intended to replace the professional medical or legal advice of a licensed practitioner.
© CIRP.org 1996-2024 | Please visit our sponsor and host: IntactiWiki.