BJU International, Volume 83, Suppl. 1: Pages 52-62, January 1999.
Medical College of Wisconsin, Department of Pediatrics, Marshfield Clinic, Lakeland Center, Minocqua, Wisconsin, USA
Despite the wide availability of condoms and the fear of HIV infection, sexually transmitted diseases (STDs) continue to be a serious public health concern. In the medical literature about preventive measures, circumcision is rarely if ever mentioned as an effective preventive measure: however, articles promoting the routine practice of circumcision invariably mention the surgery's benefit of reducing STDs. One author refers to over 100 medical articles supporting this thesis [1]. In the present review, the medical literature is examined to determine what influence, if any, circumcision has on STDs.
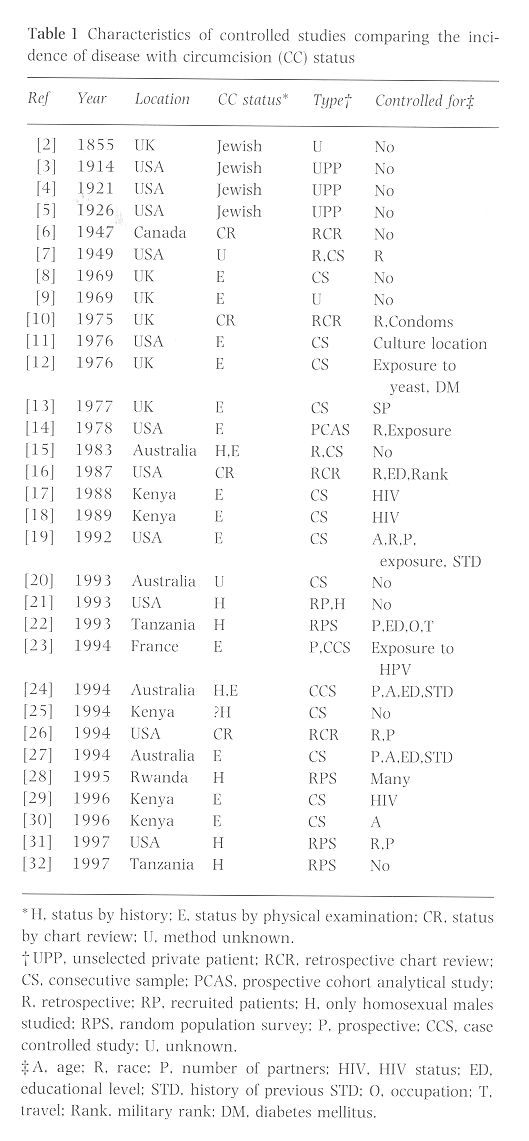
A Medline search was conducted using the keyword 'circumcision' and titles located were examined to determine if they applied to the topic. Other articles were identified through cited studies. Studies with identifiable control groups were included for analysis. Only 31 studies met this criterion [2-32]. Where data were available, odds ratios (ORs) and 95% CI were calculated; an OR of > 1.0 denotes a positive correlation with the presence of a foreskin.
Methodological differences
The reliability of the control group is a problematic issue. For example, several studies used men with no STD as controls, without controlling for the number of sexual partners. It is likely that men in the control group had fewer partners and thus less exposure risk, making them inappropriate controls. In Hand's 1949 study [7], Jews made up 17% of the entire study population, but only 2.2% of the general publication [33]. Blacks in Hand's control group were more likely to be circumcised than control white Gentiles (OR 1.41, 95% CI 0.87-2.27), which conflicts with reliable published data [31]. Some studies combined men with no particular STD to form control groups. Some studies considered only men with a different STD as the control group. Where the data were available, results are reported in this review using a control group of men with a STD other than the one in question, in an attempt to control for exposure risk.
Most of the studies before 1994 made no attempt to control for known confounding factors such as race, age, socio-economic status, level of education, number of lifetime sexual partners, frequency of sexual contact, or previous STD. The importance of these factors cannot be understated. For example, Laumann et al. [34] found that as a man increases the number of sexual partners, the risk of contracting STD does not increase linearly, but nearly exponentially. Without controlling for these factors, the results of these studies are of limited value and make it impossible to determine whether circumcision status is a marker for a significant risk factor or whether circumcision status isthe risk factor.
Study type
Nearly all of the studies published to date used information collected at STD clinics. While an STD clinic will concentrate clinical material at one location, study populations and controls derived from the clientele of STD clinics may not reflect the population as a whole, and may introduce a population bias that could unduly influence the results generated [34]. For example, Wilson compared seasoned soldiers who had STDs with new recruits [6]. To generalize the results of studies conducted in STD clinics, it must be assumed that circumcised men use these health facilities in the same manner. However, this is not the case, as socio-economic and cultural factors often differ between these groups. In the USA, men of higher socio-economic status are more likely to be circumcised and more likely to have an STD treated by a physician in private practice rather than at an STD clinic. Circumcised men may exhibit different health-seeking behaviour than uncircumcised men, being more likely to seek care for minor abrasions, thus being placed in the control group more often than their uncircumcised cohorts [35]. This would cause the association between the foreskin and various STDs, including HIV infection to be overestimated, if not spuriously generated [36]. In one African study, circumcised men had more lifetime partners, were more likely to smoke, drink alcohol, or have contact with prostitutes. They were less likely to be married [30]; none of these factors were controlled for in that study.
The most reliable type of study is a random population survey; to date only a couple have been published [28,31,32]. The drawbacks of these studies is that more subjects are needed to make a significant difference and circumcision status is determined by history, but the lack of population bias may compensate for these shortcomings.
Circumcision status
Cook et al. [26], in their retrospective chart review, found that circumcision status was documented in 86% of clinic notes. Taylor and Rodin recorded circumcision status in 89% of their charts [10]; these percentages are strikingly high. The Mayo clinic could not identify circumcision status in 16% of their casesof penile cancer [37].
Parker et al. [15] found a high correlation between history and physical examination, with a sensitivity (Sen) of 98%, specificity (Sp) of 99%, a positive predictive value (PPV) of 99%, and a negative predictive value (NPV) of 98%, while other studies have found no correlation. In a study in 1960 of men in New York and Los Angeles, only half of circumcised men correctly identified themselves as circumcised (Sen 51%, Sp 96%, PPV 93%, NPV 35% [39], men in Latin America (Sen 89%, Sp 84%, PPV 41%, NPV 98%) [40], and men in Africa (Sen 94%, Sp 72%, PPV 69%, NPV 95%) [32] yielded similar results.
Before the development of the germ theory of disease and modern epidemiology, it was noted that Jews had a lower incidence of syphilis [41], but a higher incidence of gonorrhoea, than Gentiles [2]. One rationale that developed to explain this finding was that Jews were protected from syphilis by circumcision. The influence of religion on sexual practices and the ban on Christian prostitutes consorting with Jews [42] were not considered in deriving this theory. Since then, the theory has been perpetuated and embellished.
Literature review
Most studies cited as supporting a role of circumcision in the prevention of STDs are not case-controlled. Several studies make unsubstantiated estimates of male circumcision rates in the general population to use for comparative purposes. Other studies use ethnicity and tribal affiliation as an indicator of circumcision status. While these measures are crude and unscientific, the available data have been used to assess risk and statistical significance. The characteristics of the studies using identifiable control groups are listed in Table 1. Most studies that were not random population surveys were conducted at STD clinics.

Normal microbial flora
The studies are summarized in (Table 2). Urethral smears of healthy uncircumcised males are less likely to have Gram-positive organisms (OR 0.40, 95% CI 0.18-0.91) [43], while Staphylococcus aureus is cultured significantly more often from periurethral swabs of circumcised boys 12 months and younger [44]. Not surprisingly, pyoderma is more common after circumcision in male infants than do those not circumcised [45,46]. Facultative Gram-negative rods and Escherichia coli are commonly found in the periurethral flora of uncircumcised males (OR 3.72, 95% CI 0.84-16.54 [43]; E. coli OR 5.52, 95% CI 2.95-10.33 [44]). What impact this has on the susceptibility to illness has yet to be determined.
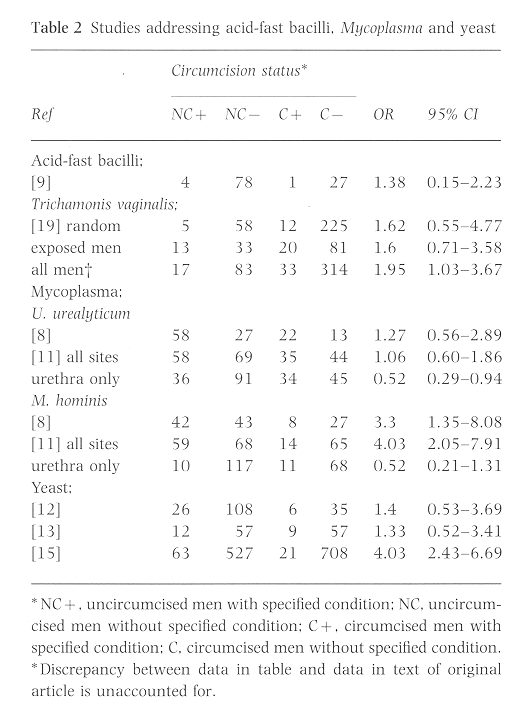
Acid-fast bacilli
It was conjectured that Mycobacterium smegmaticus could be a cause of urethritis. A study looking for acid-fast bacilli in urethral smears of men with urethritis failed to detect circumcision status as a significant factor (OR 1.38, 95% CI 0.15-2.23) [9].
Mycoplasma
Controversy exists over whether Ureaplasma urealyticum and Mycoplasma hominis are normal flora or STDs. While some studies have found these organisms more commonly in men with urethritis, others have found their prevalence to be nearly identical in men with urethritis and asymptomatic controls [8,11]. A few studies have investigated circumcisions impact on the prevalence of mycoplasmas. One of the significant variables is the location of the organisms. Both U. urealyticum and M. Hominis were more likely to be obtained from the coronal sulcus in men with foreskins. When only urethral swabs are considered, U. urealyticum is more common in circumcised men [11]. A change in sub-preputial flora takes place with the onset of sexual activity, making this flora more like that of the adult female vagina. It has not been shown whether organisms cultured from the coronal sulcus affect urethral flora or disease.
Trichomoniasis
Krieger et al. [19] found that uncircumcised men were more likely to have Trichomonas vaginalis detected on a urethral swab or first-void urine cultures (OR 1.95, 95% CI 1.03-3.67). However, when logistic regression is applied (controlling for age, race, age at sexual debut, exposure to T. vaginalis, number of sexual partners, and previous treatment for T. vaginalis, gonorrhoea, or non-specific urethritis), circumcision status was not a significant factor. (OR 1.1, 95% CI 05--2.3).
Yeast
Of studies looking for the presence of yeast, Rodin and Kolator [12] and Davidson [13] detected no difference in carriage of yeast between those with and without a foreskin. However, circumcised carriers were more likely to be asymptomatic, making these men a more serious vector for the spread of yeast infections to women. [13]. It is likely that Parker et al. only cultured swabs from symptomatic men for yeast [15]. This lack of random sampling may explain their findings.
Genital ulcer disease vs. urethritis
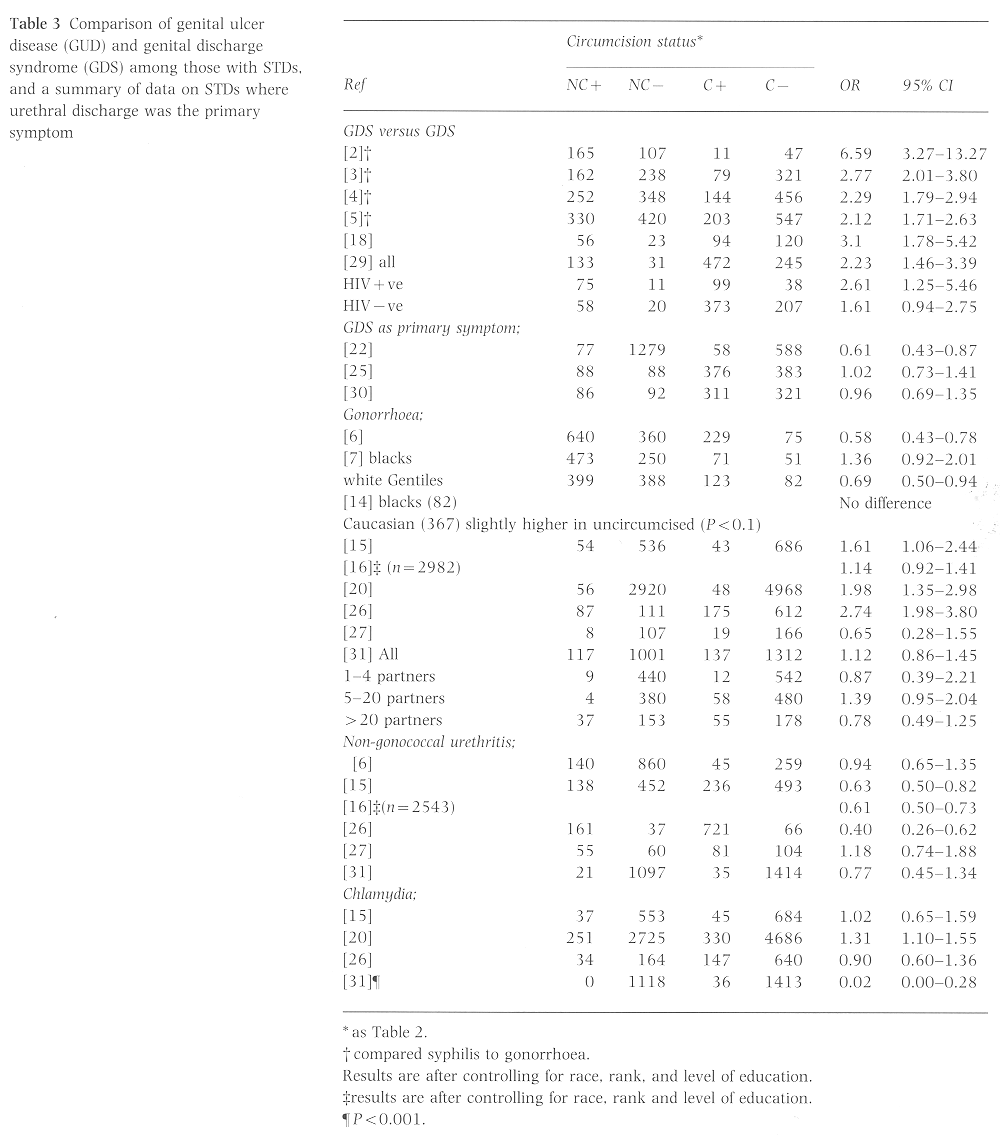
Instead of comparing individual disease entities, several studies have compared genital ulcer disease (GUD), which includes syphilis, chancroid, herpes simplex, etc., to urethritis, which includes gonorrhoea, chlamydia, and urethritis (Table 3). Instead of using a control group, these studies included those with either GUD or urethritis and compared the two entities. Similarly, the earliest study suggesting a protective role for circumcision found that Jews in London were more likely to get gonorrhoea and Gentiles were more likely to get syphilis (OR 6.59, 95% CI 3.27-13.27) [2]. Wolbarst likewise found that circumcised men were more likely to present with gonorrhoea than with syphilis [3-5]. It can be gathered from these studies that circumcised men presenting with an STD are more likely to have urethritis, while uncircumcised men are more likely to present with GUD. Because there is no true control group, no inference regarding disease prevention can be drawn from this information.
Urethritis
In the random population survey addressing urethritis, uncircumcised men were less likely to have it (OR 0.61, 95% CI 0.43-0.87; Table 3). The authors noted that most cases of urethritis is that region were from gonorrhoea [22]. Two STD clinic studies from Africa failed to document a difference [25, 30].
Gonorrhoea
Most of the data for the incidence of gonorrhoea have come from STD clinic studies (Table 3); the results have been inconsistent. When appropriate control groups are applied to the studies from the 1940s [6,7], circumcised white men are at higher risk for gonorrhoea. When the data from Smith et al. were categorized by race, no difference in gonorrhoea rates could be detected between those men with and without a foreskin [16]. In a study of 537 sailors examined for gonorrhoea before and after sexual exposure during shore leave in the Far East, circumcision status did not affect susceptibility in blacks, and although uncircumcised whites had higher rates of gonorrhoea than circumcised whites, the difference was not statistically significant (P>0.10) [14]. In a random population survey, Laumann et al. [31] discovered that the number of lifetime partners dramatically affected the impact of circumcision on gonorrhoea. While circumcised men with 5-20 lifetime partners were at lower risk for gonorrhoea, circumcised men with <20 lifetime partners were at significantly higher risk of gonorrhoea than uncircumcised men with a similar number of lifetime partners. It may be that more partners increases the diversity of the subpreputial flora, thus offering some protection to the uncircumcised male. [47].
Non-gonococcal urethritis
Non-gonococcal urethritis (NGU) is not prevented by circumcision and may be more common in circumcised men (Table 3). Some of the studies distinguished between chlamydia and NGU [15,26,31]. A significant correlation between the foreskin and NGU has yet to be detected and a negative correlation appears likely. Chlamydia is most commonly the cause of NGU: the data suggest that circumcised men are at greater risk of chlamydial infections.
Genital ulcer disease
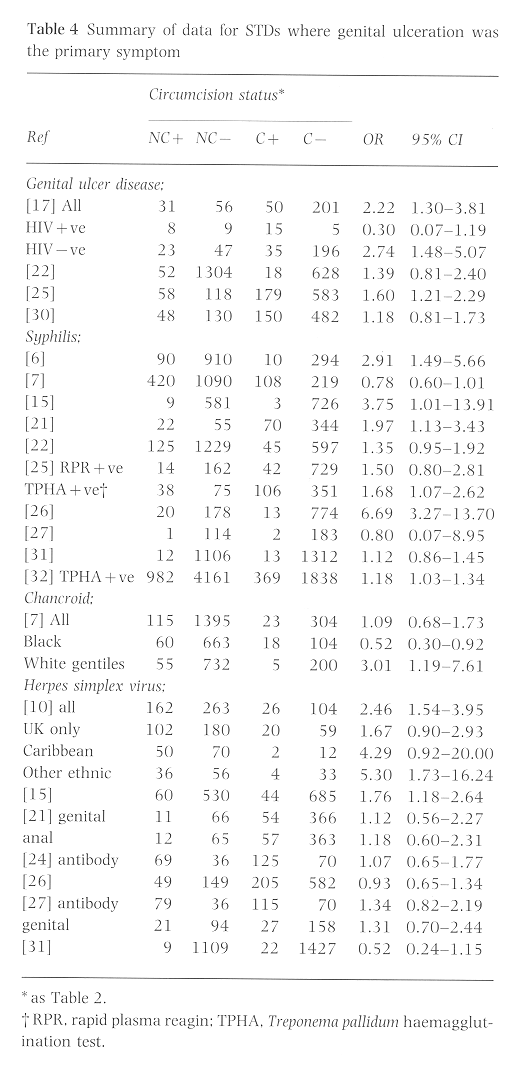
Several of the HIV studies examining the role of circumcision have also provided data on its role in the incidence of GUD (Table 4). The findings have been inconsistent but suggest that uncircumcised men may be at greater risk for GUD.
Syphilis
One of the difficulties in studying syphilis is its low prevalence. Most of the recent studies have been unable to detect a statistically significant difference because there are too few cases. Even the large population survey by Laumann et al. could not detect a difference [31]. The results by Kreiss and Hopkins [21] cannot be generalized to the population at large because both circumcision and syphilis status were obtained by history from a study population that only included men engaging in homosexual activity. When the African data reported in a study by Urassa et al. [32] are controlled for background characteristics, including HIV status, no difference is detectable (OR 1.05, 95% CI 0.97-1.27).
Chancroid
Circumcision was once proposed as a treatment for chancroid [48,49] until antibiotics were shown to be effective [50]. Although it is generally reported that chancroid is more common in uncircumcised males [51-55], a review of the medical literature found no case-controlled study to support this assertion, other than the study by Hand [7]. When the proper control group is applied to Hand's data, an interesting finding emerges. Chancroid is significantly more common in uncircumcised white males, but significantly more common in circumcised black males. In two studies from Singapore STD clinics, Malays, who are predominantly Islamic, were less likely to have chanchroid, but in neither study was the finding statistically significant., as there were too few Malays [56,57]. In one of the studies, Indians were more likely to develop chancroid than Chinese (OR 20.77, 95% CI 8.11-53.17). When Malays were compared with Chinese, Malays had a higher incidence of chancroid (OR 3.12, 95% CI 0.81-12.08) [56]. In both studies, calculations were based on assumptions of ethnic prevalence in the population and ethnic associations with circumcision status. If chancroid is related to circumcision status, Malays, who routinely circumcise, would be expected to have a lower incidence of chancroid than the Chinese who do not.
In an outbreak of chancroid in Winnipeg, Manitoba, Canada, the community of Native Indians and MÉtis (mixed Indian and Caucasian) were the focus of the outbreak, rather than Caucasians [52]. The published evidence suggests that race and ethnicity are more likely to be a factor in chancroid infection than circumcision status. This finding is consistent with the recognized tendency of highly contagious STDs to concentrate in 'core' populations [34]
Herpes simplex
The results for genital herpes have been inconsistent. Hand, without providing data, reported that circumcision had no impact on the incidence of genital herpes [7]. When Taylor and Rodin's data are analysed by race, the significance of the foreskin for those originally from the UK disappears [10].
Human papillomavirus and genital warts
Much attention has been paid to human papillomavirus (HPV) since it was linked with cervical and penile cancers [37,58] (Table 5). The epidemiology of these cancers mimics that of STDs. Genital warts have consistently been a significant risk factor for penile cancer [59,60]. The role of circumcision in the prevention of penile cancer is controversia, especially in light of a recent report from Seattle in which 42% of men with penile cancer were circumcised [60]. In recent studies, HPV-associated lesions are either equally common [15,23,27] or more common in circumcised men. A larger cohort of circumcised men in the USA is reaching the age at which penile cancer occurs. This has resulted in more circumcised men with penile cancers than seen previously [61,62]. How the higher risk of genital warts in circumcised men will affect penile cancer has yet to be determined. The use of condoms and screening men at risk for HPV infection using acetic acid and colposcopy may be the best use of resources for preventing penile and cervical cancers. [63,64].
Hepatitis
In an Israeli study of the prevalence of hepatitis B surface antigen in 9182 school children, the investigators speculated as to whether male circumcision could be an important mode of infection, explaining both the male predominance and the striking seasonal trend among boys that their study revealed [65]. An Ethiopian study suggested that circumcision may play a role in HBV transmission [66], but a Gambian study failed to find an association [67]. Likewise, Donovan et al. [27] and Laumann et al. found no difference in hepatitis between men with andwithout a foreskin.
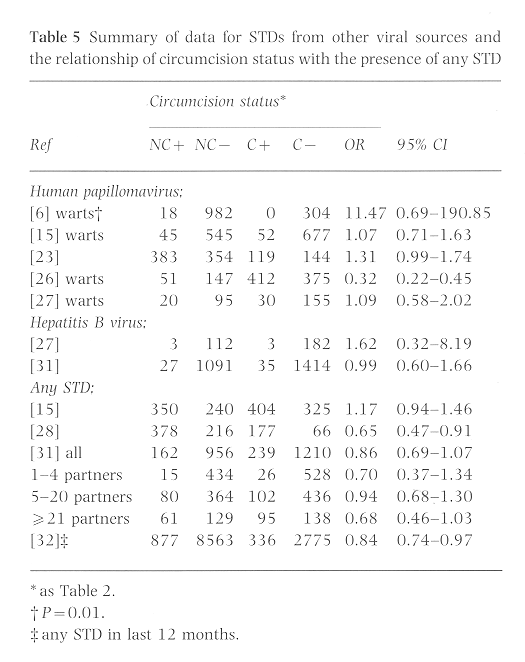
STD prevalence
Until recently, no studies have examined the impact of circumcision on overall STD incidence. The data indicate that circumcised men may be a higher risk for an STD (Table 5). This is consistent with trends seen in the USA. As routine neonatal circumcision has been implemented, the rate of STDs has increased rather fallen. Among first-world nations, the USA has one of the highest rates of STDs. HIV infection and male circumcision. In the report by Cook et al. [26] the average uncircumcised man had 2.16 STDs diagnosed, while in the average circumcised man it was 2.32; in an Australian study, there was no difference (1.48 vs 1.44 STDs diagnosed per patient) [27].
HIV
There have been 36 case-controlled studies published in peer-reviewed journals addressing the relationship between the foreskin and HIV infection; the results have been inconsistent. Several studies performed in STD clinics have found the foreskin to be a risk factor [17,18,21,25,29,30,68-72], while several random population surveys, which do not have the population bias of an STD clinic study, have found circumcised men to be at higher risk [32,73-75]. Several studies have failed to detect a statistically significant difference between men with and without a foreskin [31,76-87]. In several studies, when the populations are controlled for GUD, number of sexual partners and other factors, the results differ significantly from the raw data. The USA has the highest incidence of HIV infection, as well as the highest incidence of male circumcision amongst developed nations. This speaks against the protective effect of circumcision [89]. The inconsistency of the results and the number of confounding factors make it impossible to link the foreskin to HIV infection [36,90].
It is impossible to assert a causal relationship based on retrospective data. Hill developed criteria for assessing whether a strong case can be made for causality based on retrospective data [91].
Strength: If an association has a high OR, it weighs in favour of a causal relationship. Few of the associations have had an OR of > 2.0. Moreover, the studies that randomly accessed portions of the population outside of STD clinics have found circumcised men to be at higher risk for an STD [28,31,32].
Consistency: Results among studies have been very inconsistent: this speaks against causality.
Specificity: There are several confounding factors: circumcision is not chose randomly [92]. Significant factors such as race, socio-economic status, education level and number of partners are often not controlled for in the published studies. In most studies where these factors have been controlled for, the foreskin is not a significant factor. For example, a US Army study of soldiers in Japan reported that uncircumcised men were more likely to have a penile lesion than were random controls (OR 22.78, 95% CI 6.00-86.29), but men with penile lesions were more likely to be black (OR 15.00, 95% CI 3.12-72-07) or to have had a previous STD (OR 4.41, 1.71-11.34) [93]. No attempt was made to control for these significant factors.
Temporality:
Most males are circumcised before beginning sexual activity. While some effort has been made to promote circumcision as an HIV preventive in Africa [94], no studies of the effects of later circumcision on disease prevention have been published.
Biological gradient: One study of HIV found that circumcised men with more residual foreskin were at higher risk of HIV-2 infection [77]. It is difficult to make any conclusion from this one study.
Biological plausibility: Moses et al. [95] suggested: (a) that minor inflammatory conditions can occur underneath the foreskin, resulting in mucosal discontinuity that may provide a portal of entry for viruses and bacteria: (b) that the foreskin may be more susceptible to minor trauma during intercourse: (c) that the warm, moist environment under the foreskin may provides an environment conducive to prolonged survival of pathogens; and (d) in the case of HIV, that Langerhans cells, plentiful in the foreskin of male macaque monkeys, are highly susceptible to simian immunodeficiency virus (SIV) [95].
While the warm moist environment under the prepuce allows for the growth of all bacteria, including pathogens, the immunological protection provided the subpreputial flora [96], secretory immunoglobulins, and lytic secretions from the prostate, urethra, and seminal vesicles [97] have not been adequately investigated. Undisturbed preputial flora and mucosal immunological defences may protect the uncircumcised male for infection [47].
The role of Langerhans cell in the transmission of HIV is unclear. While they are present in the mucosal prepuce of monkeys [98] and adult males [99], Weiss et al. were not able to detect their presence on the inner surface of prepuces taken from newborns [100]. These cells initiate the immune response to infectious agents. In the primate study referred to by Moses et al. [95] it was Langerhans-like cells in the lamina propria, and not in the epithelium, that appeared to be infected with SIV [101]. It is unclear whether this observation can be extrapolated to the Langerhans cells in the epithelium of the prepuce in humans.
Beaugé suggests that the loss of penile skin from circumcision frequently results in tightened skin over the erect penis. This increases friction during intercourse and increases the likelihood of abrasions through which a pathogen can be introduced systemically, making the circumcised penis more likely to contract an STD [101]. The increased likelihood of circumcised men engaging in active anal sex [31] may also increase a circumcised man's susceptibility to STDs.
Coherence:
Circumcision as a preventive measure for STDs needs to fit into a coherent explanation that takes other know risk factors into account. To date, the only theory proposed is the 'subpreputial space as a cesspool' explanation provided by Weiss [103]. If the 'cesspool" theory is extended to women, then one would expect that women, who have significantly more genital mucosa, would have markedly more STD's than men, whether circumcised or not; this is not the case [34]. Nearly all of the associations between STDs and the foreskin can be explained on the basis of racial, socio-economic, cultural, ethnic, and healthcare-seeking behavioural differences. Studies controlling for these factors have failed to confirm the efficacy of circumcision in preventing STDs.
Experimental evidence:
There is none.
Analogy:
Is there another illness to which the foreskin makes a man more susceptible? Can it be equated with the acquisition and transmission of STDs? Lower urinary tract infections in infancy may be associated with the foreskin, but showing analogy to STDs would be a formidable task. Upper urinary tract infections in males are most often related to anatomical anomalies in the male rather than the foreskin [104], and unrelated to behaviour patterns. The aetiologies of these maladies are so disparate that forming an analogy is impossible. Based on the above criteria, a causal relationship between the foreskin and STDs cannot be inferred.
What began as speculation has resulted a century later in 60-75% of American boys being circumcised with no clearly confirmed medical benefit. In the interim, no solid epidemiological evidence has been found to support the theory that circumcision prevents STDs or to justify a policy of involuntary mass circumcision as a public health measure. While the number of confounding factors and the inability to perform a random, double-blind, propective trial make assessing the role of circumcision in STD acquisition difficult, there is no clear evidence that circumcision prevents STDs. The only consistent trend is that uncircumcised males may be more susceptible to GUD, while circumcised men are more prone to urethritis. Currently, in developed nations, urethritis is more common than GUD [34]. In summary, the medical literature does notsupport the theory that circumcision prevents STDs.
Circumcision: foreskin fact, foreskin fictionPresented at Advances and Controversies in Clinical Pediatrics, San Francisco, California, May 17, 1997: cited in Boschert S. Giving parents the facts on circumcision. Pediatr News 1997; 31: 40
non-specificgenital infection. 3. Post-gonococcal urethritis. A prospective study. Br J Ven Dis 1969: 45: 282-6
Author
R. S. Van Howe, MD, FAAP, Clinical Instructor.
Medical College of Wisconsin, Department of Pediatrics,
Marshfield Clinic, Lakeland Center, 9601 Townline Road,
PO Box 430, Minocqua, Wisconsin 54548-1390, USA
E-mail: vanhower@gabby.mfldclin.edu
The Circumcision Information and Resource Pages are a not-for-profit educational resource and library. IntactiWiki hosts this website but is not responsible for the content of this site. CIRP makes documents available without charge, for informational purposes only. The contents of this site are not intended to replace the professional medical or legal advice of a licensed practitioner.
© CIRP.org 1996-2024 | Please visit our sponsor and host: IntactiWiki.